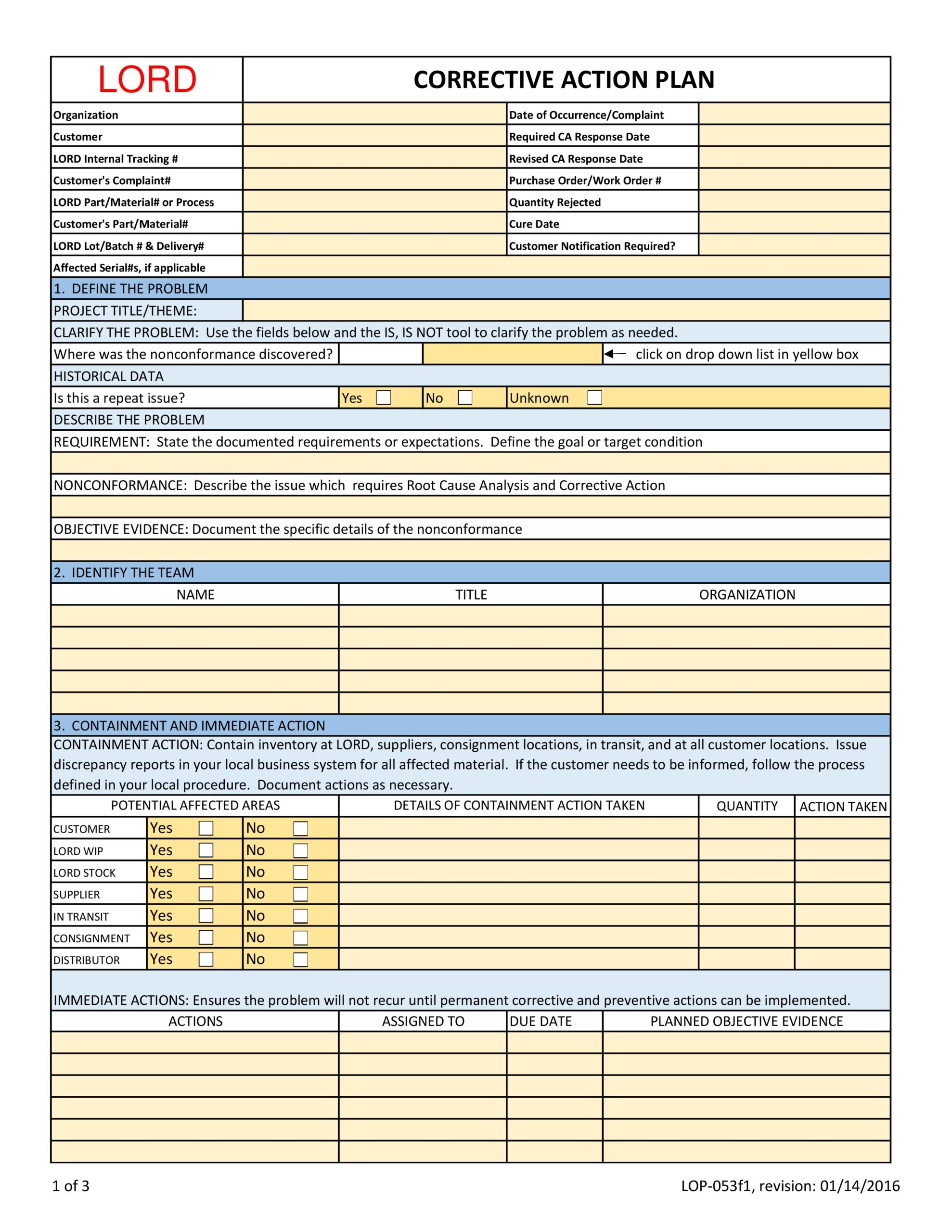
Template SOP Corrective and Preventive Action CAPA , ISO 13485 2016 Section Document Section 8 5 1 All 8 5 2 All 8 5 3 All Summary This SOP describes how CAPAs are implemented and tracked Process Owner enter role of process owner Key Performance Indicators enter KPIs to be tracked for the Management Review Process Steps 1 Input for CAPA Various events may lead to creation of CAPA Examples . Corrective and Preventive Action CAPA Form SimplerQMS, Corrective and Preventive Actions CAPA Form Template Our free CAPA form template has all the required fields and is a quick readymade solution for busy life science professionals who are looking to save time All you need is to fill the fields with essential details signatures and dates
.Capa Form Template Free
Capa Form Template Free
Template SOP Corrective and Preventive Action CAPA
ISO 13485 2016 Section Document Section 8 5 1 All 8 5 2 All 8 5 3 All Summary This SOP describes how CAPAs are implemented and tracked Process Owner enter role of process owner Key Performance Indicators enter KPIs to be tracked for the Management Review Process Steps 1 Input for CAPA Various events may lead to creation of CAPA Examples .
Corrective and Preventive Action CAPA Report SimplerQMS
The CAPA form is completed with all the relevant approvals and signatures In an eQMS all of the relevant approvals can be routed through an automated workflow and electronic Part 11 Compliant signatures can be captured See how electronic signatures and automated workflows work in SimplerQMS CAPA Report Form Template Free Download .
Corrective Action Preventive Action CAPA Form Free Download
Free downloadable CAPA template for medical devices The CAPA form provides a means to document the correction or prevention of non conformances related to elements of the quality system CAPA is an important part of continuous improvement A robust CAPA program supports enhanced product quality .
Free CAPA Template for Medical Devices Downloadable
Free downloadable CAPA template for medical device companies to streamline and improve the corrective and preventive action process A Free CAPA Template for the Medical Device Industry Written by Jon Speer September 12 2024 Share .
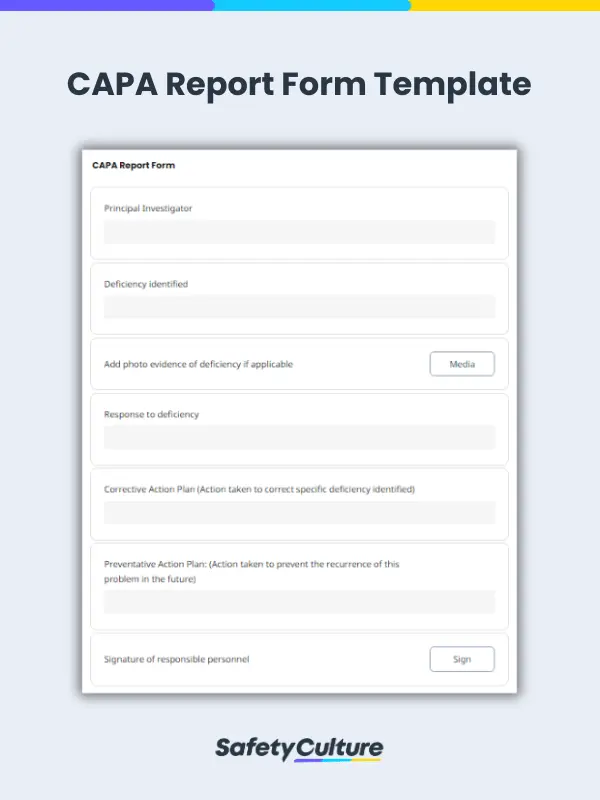
Free CAPA Report Template Editable Free Download Lumiform
CAPA report template An effective tool for root cause analysis and corrective actions Use this change order form template for requesting approving and documenting alterations to the project 10 free quality audit checklists 6 free quality management plan templates 17 free scope of work templates Problem solving with an A3 report .
Corrective and Preventive Action CAPA Form Template SimplerQMS
CAPA form is a tool you can use to initiate any non conformance issue or deviation customer complaint audit finding or any other quality event into the CAPA process It will be initiated as soon as the CAPA request is accepted and converted into a formal CAPA This form tracks all corrective and preventive actions taken for a given quality .
Disclaimer: The pictures showed on this website are owned by their copyright owners. Connect to us with any concerns about credit rating or elimination.